ABOUT HIARY
19
2025
-
08
[CNAS Accredited Process Safety Laboratory] Take a tour of Wuhan Hiteck Biological Pharma Process Safety Laboratory - Reaction Risk Assessment
Category:
Company News
About Us
The Process Safety Laboratory of Wuhan Hiteck Bio-Innovation Pharmaceutical Research was established in April 2022. The laboratory is dedicated to conducting process thermal safety testing and reaction risk assessment, equipped with state-of-the-art testing instruments and data processing software systems, as well as a team of professional technical experts. The laboratory is equipped with the following instruments: Reaction Calorimeter RC1, Adiabatic Accelerated Calorimeter ARC, Differential Scanning Calorimeter DSC, and Thermogravimetric Analyzer TGA, among others. In July 2023, the laboratory successfully passed the accreditation review conducted by the China National Accreditation Service for Conformity Assessment (CNAS) and obtained the CNAS Laboratory Accreditation Certificate. This signifies that the laboratory's management and technical capabilities have been recognized at both national and international levels, and it possesses the technical capability to conduct testing in accordance with relevant accreditation criteria. Process safety and risk assessment are key components of process development and an important aspect of intrinsic safety in production.
The laboratory's research team possesses extensive technical capabilities and experience in chemical reaction risk assessment, process optimization, and thermodynamic analysis. By conducting comprehensive reaction risk studies on fine chemical processes, the team can quantify process risks and provide practical safety technical data for process design, such as heat release quantities, heat release rates, and heat accumulation—parameters indispensable in process design and production. Additionally, the team can optimize processes to develop safer production methods.
01 Instrumentation and Equipment

02 Background Introduction
The former State Administration of Work Safety issued the “Guiding Opinions on Strengthening the Safety Risk Assessment of Fine Chemical Reactions” in 2017, which clearly stipulated that thermal stability tests should be conducted on raw materials, intermediate materials, and products involved in chemical reactions, thermodynamic and kinetic analyses should be performed on the chemical reaction process, and the reaction hazard level should be assessed based on parameters such as reaction heat and adiabatic temperature rise. assess the likelihood of reaction runaway based on parameters such as the time to reach maximum reaction rate, and conduct a multi-factor hazard assessment in conjunction with relevant reaction temperature parameters to determine the hazard level of the reaction process.
1) Assessment of the heat of decomposition of substances
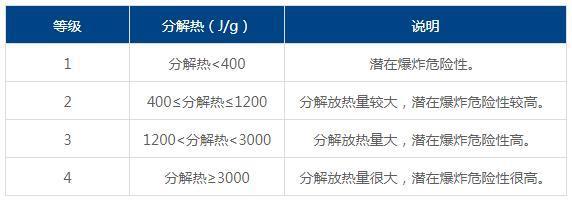
2) Response risk assessment
i) Severity assessment
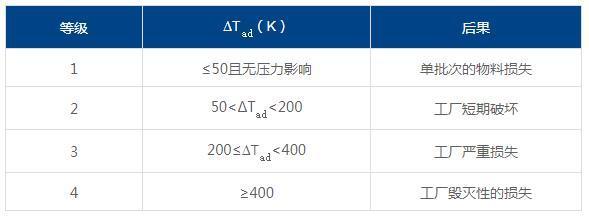
ii) Possibility assessment
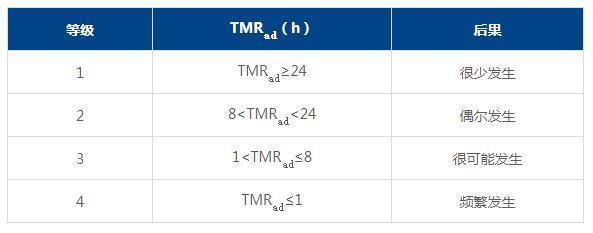
iii) Matrix assessment (Risk = Severity × Likelihood)
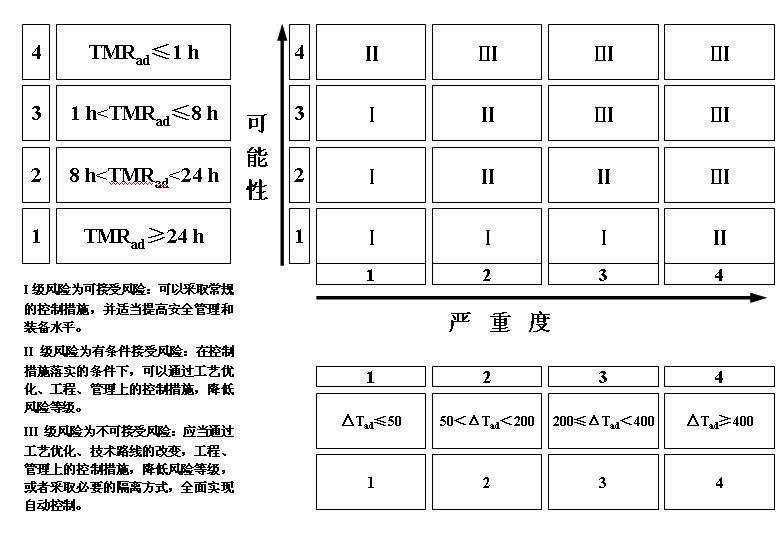
3) Reaction process hazard assessment
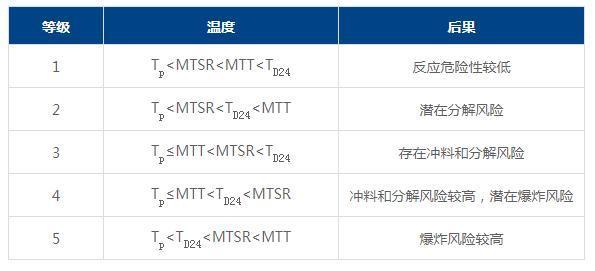
For different levels of reaction process hazard, different risk control measures need to be established. For processes with a hazard level of 3 or above, it is necessary to further obtain parameters such as the relationship between uncontrolled reaction temperature, uncontrolled reaction system temperature and pressure, maximum temperature during the uncontrolled process, maximum pressure, maximum temperature rise rate, maximum pressure rise rate, and adiabatic temperature rise, and determine the corresponding risk control measures.
03 Assessment Process
Generally, the following aspects (not limited to) are studied to assess and develop safer process routes:
1. Conduct thermal stability testing on chemicals involved in the reaction, such as raw materials, intermediate materials, and products, to obtain the exothermic decomposition conditions of the substances and conduct thermal stability assessments;
2. Assess the severity of an uncontrolled exothermic reaction through testing of temperature rise during the reaction;
3. Assess the likelihood of a runaway reaction occurring by testing the time it takes for the reaction rate to reach its maximum;
4. Assess the hazard level of the reaction process by testing the process temperature, the maximum temperature during synthesis, the temperature at which the reaction rate reaches its maximum within 24 hours, and the maximum temperature due to technical reasons;
5. Based on the assessment results, optimize high-risk processes and develop safe process routes.
04 Service Projects
Chemical Reaction Safety Risk Assessment
In accordance with the requirements of the “Guiding Opinions on Strengthening the Assessment of Fine Chemical Reaction Risks” issued by the State Administration of Work Safety [Safety Supervision General Office No. 1 (2017)], conduct a reaction safety risk assessment for organic synthesis reactions.
Safety Analysis of Hazardous Chemicals/Reaction Heat
Including:
Melting point, initial decomposition temperature, and decomposition heat release of the substance;
Real-time changes in temperature, pressure, and volume during the reaction;
Heat accumulation and kinetic data of the reaction.
3. Optimization of Chemical Processes in the Pharmaceutical and Fine Chemical Industries
Monitor changes in parameters such as temperature and pressure during chemical reactions, physical mixing, distillation, crystallization, etc., optimize process conditions, and enhance the inherent safety of production.
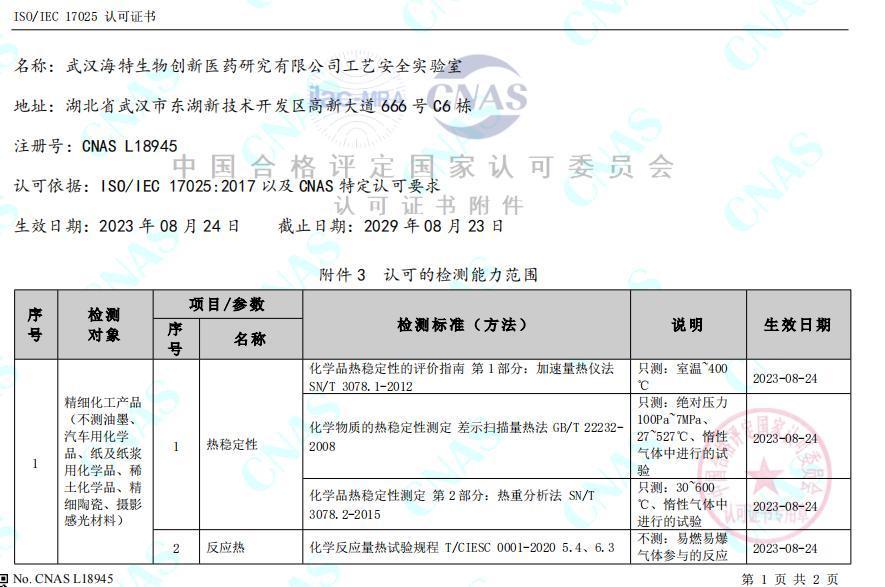
05 Qualification Documents

Related news
12-03
2025



